وارمر نوزاد
وارمر نوزاد وسیله ایست جهت تامین گرمای مکمل مورد نیاز نوزاد در بدو تولد .
دارای تختی است جهت قرار گرفتن نوزاد بر روی آن هستند و گرما معمولا از بالا به صورت تابشی بر بدن نوزاد تابیده می شود و دستگاه دارای سنسور دما بوده و اغلب به صورت اتومات کنترل می شود. این تخت دارای دسترسی آسان به بیمار بوده که درصورت نیاز ، تیم پزشکی به آسانی به کودک دسترسی خواهند داشت و اقدامات اورژانسی را به راحتی انجام خواهند داد و کودک را از مرگ احتمالی نجات خواهند داد.
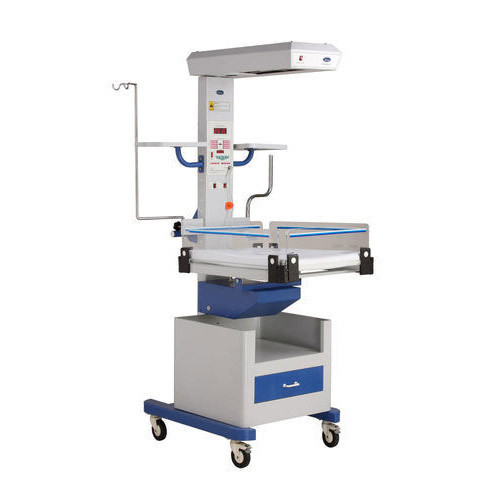
وارمر نوزاد
1– دارای سنسور دما جهت سنجش دمای بدن کودک
2- کنترلر دما جهت کنترل دقیق دمای تابیده شده
3- دارای سیستم روشنایی جهت مشاهده وضعیت ظاهری کودک
4- هشداردهنده دمای بیش از حد مجاز جهت جلوگیری از خطرات احتمالی
5- قابلیت تنظیم ارتفاع
نوزادان درصد بالایی از حرارت بدن خود را از طریق تشعشع حرارت به محیط اطراف از دست می دهند. در برخی شرایط کلینیکی نیاز است تا سطح بزرگی از بدن نوزاد بدون پوشش باشد تا پرسنل بیمارستان بتوانند شرایط بدنی نوزاد را مشاهده و همین طور وسایل درمانی به بدن نوزاد وصل شود. در این حالت از گرم کننده های تابشی و یا همان وارمر ها استفاده می کنند که با یک میکرو کنترلر حرارت کنترل می شود.
این دستگاه تشکیل شده از یک المنت که گرمای مورد نیاز را تأمین می کند. و قابلیت کم و زیاد کردن دمارا دارد. این دما با استفاده از سنسوری که به بیمار وصل می گردد مشخص می شود. نوزاد را پس از آن که از وارمر جدا کردند به داخل انکوباتور هدایت می کنند.
Health problem addressed
In under-resourced settings, hypothermia at birth is one of the most important risk factors for newborn morbidity and mortality. 99% of newborns that die globally are in such settings. It is vital to keep newborns warm and help them achieve thermoregulation in order to prevent and minimize morbidities and mortalities due to hypothermia.

وارمر نوزاد
Product description
The device consists of a biocompatible bed on which to place the infant, and an overhead heater that delivers radiant heat. A skin temperature probe monitors infant temperature. Heat output can be controlled manually or through baby mode (feedback mode) for thermoregulation. Visual and audio alarms are present for safety. An APGAR timer helps to efficiently take APGAR scores post-delivery .
Developer’s claims of products
benefitsCurrent radiant warmers have heaters typically made from quartz or ceramic. These heaters tend to breakdown faster as well as take a much longer time to heat up – up to 13 minutes in some cases. Each additional minute of cold stress can lead to increased morbidity for an infant.
This device gives better clinical results because it provides uniform heating across the bed as well as a much faster warmup time (4 minutes only) that reduces the time to warm a hypothermic infant.
Furthermore, the lower power consumption and long life of the heating element (Calrod heater) lead to considerable cost savings .
Suitability for low-resource settings
The device uses less power at startup and during operation compared to other radiant warmers. It is designed for infection control (e.g. non-stitch biocompatible mattress for no infections in stitches plus no need for a mattress cover).
It has a faster warmup time for high volume environments with little pre-warming. Over 1500 warmers have been deployed in low-resource settings.
The device has been designed, developed and manufactured in India based on extensive customer input in India and similar countries. It has been adopted in Tier 2 and 3 towns in India, as well throughout the country of Myanmar by the Ministry of Health. Testing of the device includes protocols that try to simulate low-resource setting issues such as voltage fluctuations, high humidity etc.
Operating steps
The device is usually pre-warmed in manual mode for at least four minutes. The infant is then placed on the bed mattress and the skin temperature probe is attached. The operator then switches to baby mode (feedback) and enters the desired baby temperature. The APGAR timer and observation light can be switched on as needed .
Regulatory status
It conforms with the requirements of Medical Devices Directive 93/42/EEC – BSI CE 0086 mark. It is also certified ISO 13485. It has US FDA 510K clearance (K121625), and it is ROHS compliant.
Use and maintenanceUser:
Physician, nurse,midwifeTraining: Initial training by manufacturer, and operator manualMaintenance: Annually by technician, engineer, or manufacturer
Environment of use
Settings: Rural, urban settings, primary (health post, health center), secondary (general hospital), tertiary (specialized hospital)Requirements: Continuous power supply (100-240V). Can withstand some fluctuations in voltage and occassional spikes, but stable power supply is strongly recommended. General cleaning supplies to disinfect after every infant are needed
Disclaimer
Eligibility for inclusion in the compendium has been evaluated by WHO and external technical advisers listed in the Acknowledgements. However, the evaluation has been solely based on a limited assessment of data and information submitted in the developers’ applications and, where available, of additional sources of evidence, such as literature search results or other publicly available information.
There has been no rigorous review for safety, efficacy, quality, applicability, nor cost acceptability of any of the technologies. Therefore, inclusion in the compendium does not constitute a warranty of the fitness of any technology for a particular purpose. Besides, the responsibility for the quality, safety and efficacy of each technology remains with the developer and/or manufacturer.
The decision to include a particular technology in the compendium is subject to change on the basis of new information that may subsequently become available to WHO. WHO will not be held to endorse nor to recommend any technology included in the compendium.
Inclusion in the compendium solely aims at drawing stakeholders’ attention to innovative health technologies, either existing or under development, with a view to fostering the development and availability of, and/or access to, new and emerging technologies which are likely to be accessible, appropriate and affordable for use in low- and middle-income countries
WHO does not furthermore warrant or represent that:
1. the list of innovative health technologies is exhaustive or error free; and/or that
2. the technologies which are included in the compendium will be embodied in future editions of the compendium; and/or that
3. the use of the technologies listed is, or will be, in accordance with the national laws and regulations of any country, including but not limited to patent laws; and/or that
4. any product that may be developed from the listed technologies will be successfully commercialized in target countries or that WHO will finance or otherwise support the development or commercialization of any such product.
WHO disclaims any and all liability and responsibility whatsoever for any injury, death, loss, damage, use of personal data, or other prejudice of any kind whatsoever that may arise as a result of, or in connection with, the procurement, distribution and/or use of any technology embodied in the compendium, or of any resulting product and any future development thereof.
Developers whose technology has been included in the compendium shall not, in any statement of an advertising, commercial and/or promotional nature, refer to their participation and/or inclusion in the compendium. In no case shall the latter use the WHO name and/or the emblem, or any abbreviation thereof, in relation to their business or otherwise


